Stability Room for Pharmaceutical Industry
TestLabs offer reliable and yet cost-effective walk in stability room solutions for pharmaceutical needs. All designs are customized according customer specifications and pharmaceutical industry requirement in Malaysia. The temperature range of the room is typically from +20°C to +60°C.
TestLabs stability chambers are designed to comply with international pharmaceutical technical requirement of simulation and reproduction of the climatic conditions for climatic stability. Beside, Testlabs able to assist client in execution of data logging according to FDA 21 CFR part 11.
TestLabs stability room or chamber are in compliance with all international requirements, include IQ, OQ, PQ qualifications, FDA 21 CFR part 11 data logging system and software, and ISO 17025 calibration.
TestLabs stability chambers are designed to comply with international pharmaceutical technical requirement of simulation and reproduction of the climatic conditions for climatic stability. Beside, Testlabs able to assist client in execution of data logging according to FDA 21 CFR part 11.
TestLabs stability room or chamber are in compliance with all international requirements, include IQ, OQ, PQ qualifications, FDA 21 CFR part 11 data logging system and software, and ISO 17025 calibration.
Technical specifications:
- Customizable capacities up to fit into your lab
- Temperature range from +20°C to +60°C
- Temperature fluctuation ±1.0°C
- Temperature heating rate ±1.0°C/min and Cooling rate ±1.0°C/min (average, no loading)
- Humidity range from 30%RH to 80%RH
- Humidity fluctuation ±3%RH
- PU insulation foam with Stainless Steel 304 internal wall panels
- Lockable test chamber door with easy-to-open double door latch
- Chamber designed with strong internal air circulation. Heating and cooling system located behind/below the false sheet of chamber and outside the working space
- Cooling system by Germany BOCK compressors
- Micro-processor controlled control system completed with touch panel
- Safety features are in place to protect over load trip, over temperature and blower failure
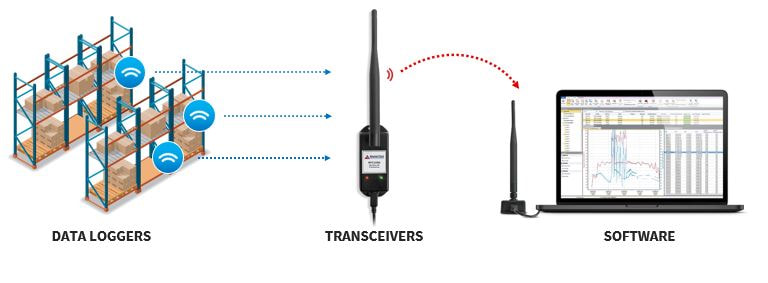
Wireless Data Logger Temperature and Humidity Monitoring System (Optional)
- Compliance to FDA 21 CFR Part 11
- Resolution: 0.01°C, 0.1%RH / Accuracy ±0.5°C, ±2.0%RH
- Data logger system sensors are made in US
- Approved by MCMC Malaysia
- Software contains criteria such as electronic signatures, access codes, secure data files, and an audit trail which meet the requirements of 21 CFR Part 11 and help provide data integrity.
- IQ/OQ/PQ execution and user training are included
Valification and Qualification IQ/OQ/PQ Protocol (Optional)
- IQ/OQ/PQ document protocol will be prepared and reviewed/accepted by client
- Temperature mapping for empty load (3 or 7 days) and loaded (3 or 7 days)
- Validation Personnel Training and Experience Records
- Pre/Post Calibration Report of Validators and Data Loggers Used
You may also like to explore this
Why Choose Us?Read our customer testimonials and our service commitment here
|